Published: 02/08/24 12:03 Categories: Microbiology
In the pharmaceutical industry, product safety and quality are of paramount importance. That is why microbiological analysis is a fundamental pillar to ensure the absence of pathogens and other microorganisms in pharmaceutical products.
Traditionally, these analyses have been based on culture methods, however, alternative methods are increasingly being adopted in other industries such as food, water, cosmetics, etc., so it seems a matter of time before they are used in the microbiological control of medicines.
Goodbye growing media, hello alternative methods!
One of the main advantages of alternative methods is their speed with techniques such as PCR, mass spectrometry and flow cytometry that can deliver results in a matter of hours, compared to the days or even weeks required by traditional methods.
This speed not only improves the efficiency of the quality control process, but also allows for faster response to potential contamination, implementation of corrective measures, and product recalls before they reach the market.
In addition, alternative methods are more sensitive and specific such as PCR, one of the most favored and implemented techniques in pathogen analysis, can detect minuscule amounts of genetic material from the target microorganism, which allows the identification of contaminants that could go undetected with conventional methods.
A crucial aspect to guarantee the quality and safety of pharmaceutical products both in the final product, during manufacturing processes and in environmental monitoring.
However, these methods also have disadvantages, including specialization in the use of the products and reading of results, and their implementation requires an investment in equipment and adaptation of spaces that can be challenging, especially in small production scales.
Future prospects according to Pharmacopoeia
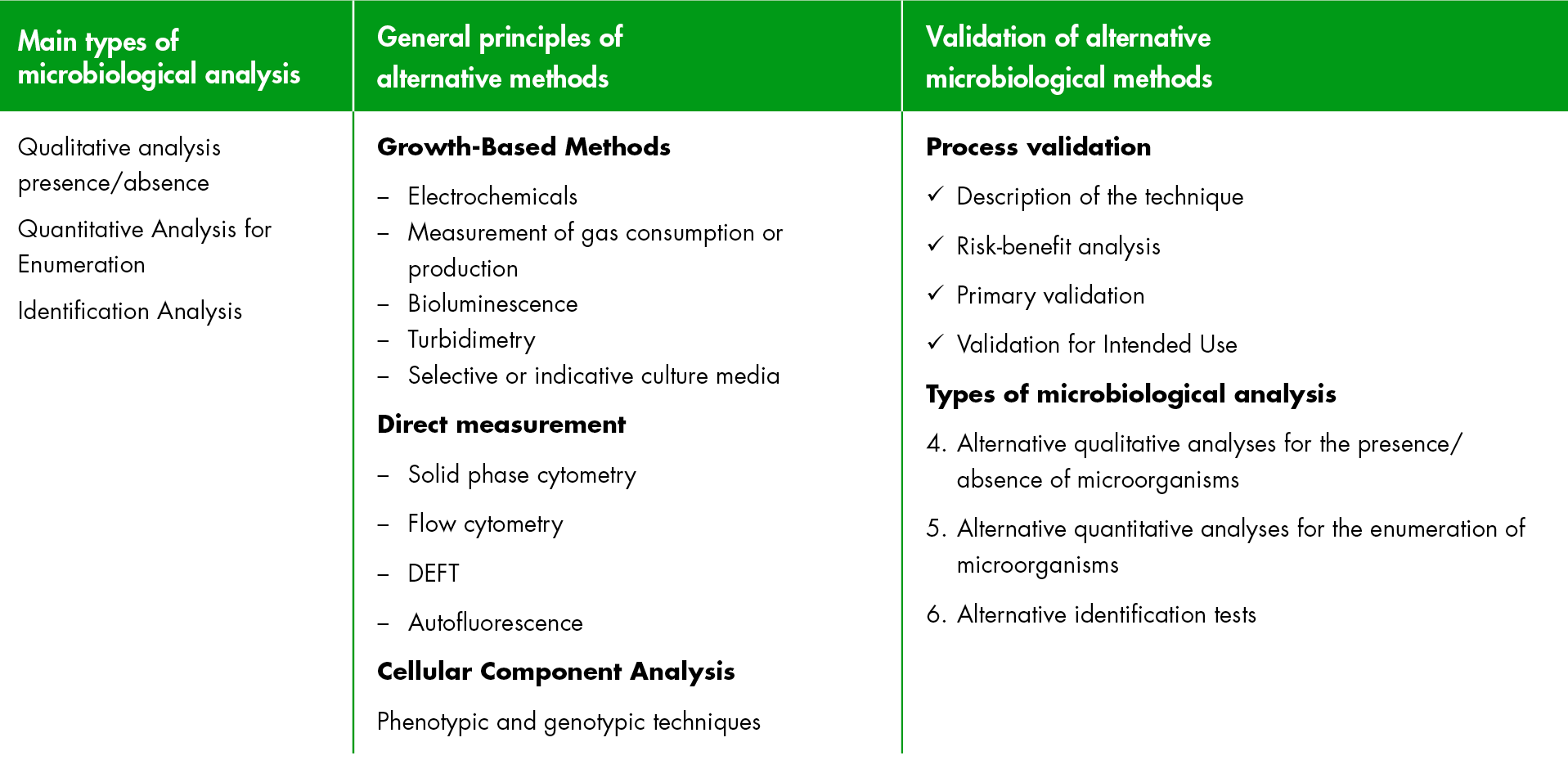
Currently the European Pharmacopoeia (Eur. Ph.) does not recommend a specific alternative method, but we can find the available methods or techniques that can be implemented as a supplement or even as an alternative to the reference methods.
They are classified depending on the type of analysis: qualitative (absence/presence), quantitative (counting), or identification.
Looking ahead, we are likely to see an increasing integration of these alternative methods into harmonized microbiological analysis protocols, both in Europe, through the European Medicines Agency (EMA), and in the US, with the FDA.
Regulation and guidelines for the implementation of these technologies will be the next step to facilitate the path for pharmaceutical companies.
In addition, with the continuous development of new technologies that improve the efficiency and effectiveness of microbiological analyses along with data analysis, we will be able to have access to even more accurate and complete solutions.
If you want to know more about the current methods according to the Pharmacopoeia or are interested in learning more about our products, we invite you to watch our webinars.

 Probiotics: Which ones are good?
Probiotics: Which ones are good?
 Condalab Says YES to the World’s Leading Lab Trade Fair: Analytica 2026
Condalab Says YES to the World’s Leading Lab Trade Fair: Analytica 2026
 CONDALAB to Exhibit at WHX Labs Dubai 2026
CONDALAB to Exhibit at WHX Labs Dubai 2026
 Food fraud: How do we detect it?
Food fraud: How do we detect it?
 Visit Us at MEDICA 2025 – Discover Our Precise Detection Solutions
Visit Us at MEDICA 2025 – Discover Our Precise Detection Solutions
